What is Diamond | Origins
| History
| Mining & Distribution| Diamond Jewels
structure
A neutral carbon atom has 6 protons and 6 electrons nearby its nucleus. Four of the electrons in a carbon atom are valence electrons, which are electrons that are obtainable to form bonds with other atoms. In graphite, each carbon atom bonds only 3 of its 4 valence electrons with its surrounding carbons. The consequential structure of these bonds is a flat sheet of connected carbon atoms. Although individually strong, these layers are only weakly connected to one another, and the ease with which they are separated is what makes graphite so slippery.
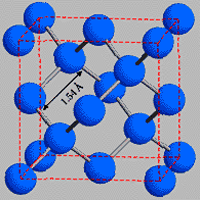 This model shows how each carbon atom (ball) is associated to 4 other carbon atoms by strong chemical bonds (rods), creating diamond's rigid crystal structure.
This model shows how each carbon atom (ball) is associated to 4 other carbon atoms by strong chemical bonds (rods), creating diamond's rigid crystal structure.
In diamond though, every carbon shares all 4 of its available electrons with adjacent carbon atoms, forming a tetrahedral unit. This shared electron-pair bonding forms the strongest known chemical linkage, the covalent bond, which is responsible for many of diamond's excellent properties. The repeating structural unit of diamond consists of 8 atoms which are basically arranged in a cube.
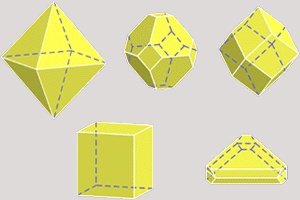
Using this cubic form and its extremely symmetrical arrangement of atoms, diamond crystals can develop in a variety of different shapes known as "crystal habits." The octahedron or eight-sided shape that we associate with diamonds is its most general crystal habit. However diamond crystals can also form cubes, dodecahedra, and even combinations of these shapes. Every single shape is manifestations of the cubic crystal system to which the mineral diamond belongs. Two exceptions are the flat form called a macle, which is actually a composite crystal, and etched crystals, which have rounded surfaces and, sometimes, extended shapes.
Next >>