What is Diamond | Origins
| History
| Mining & Distribution | Diamond Jewels
Electrical Conduction
Why does graphite conduct electricity whereas diamond does not? The difference in their appearances is one clue. The somewhat metallic shine of graphite announces its capacity to conduct electricity. Metals conduct electricity, while transparent substances, with diamond, are poor electrical conductors. Though, rare diamonds, particularly the gray-to-blue ones called type IIB, are semiconductors, and are somewhat conductive. Both diamond and graphite have important applications in electronics: Diamond may be used as either an insulator (nonconductor) or a semiconductor, and graphite is generally used as a conductor.
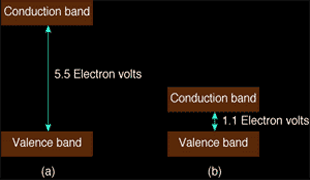
A substance is an electrical conductor if very little energy is necessary to move an electron from its bonded condition -- in the so-called valence band -- to a mobile condition in the conduction band. Such conductors are metallic looking since light is moving the electrons, too. In nonconductors (insulators) a huge energy barrier exists between the valence band and the conduction band. This barrier should be "jumped" to allow conduction. Semiconductors such as kind IIB diamonds have impurities that shrink the energy jump to the conduction band. These diamonds are reasonable conductors of electricity.
<< Back | Next >>