What is Diamond | Origins
| History
| Mining & Distribution | Diamond Jewels
Density
Density is a ratio of a substance's accumulation to its volume. For example, density explains why a certain amount of lead feels heavier than an equal volume of salt. Diamond is astonishingly dense given the low atomic weight of carbon. At 3.51 grams per cubic centimeter, diamond is much denser than graphite, which weighs in at only 2.20 grams per cubic centimeter. This comparison offers an important sign to diamond's origin: the fact that diamond's carbon atoms are "squeezed" together tighter than in graphite, which forms near Earth's surface, implies that diamond is formed under high pressure conditions. This model was corroborated by the experimental synthesis of diamond at high pressure and temperature illustrated on the graph below.
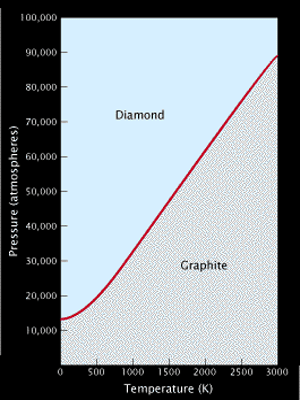
This easy diagram shows the conditions of pressure and temperature where diamond and graphite will be the stable forms of carbon. The points show the conditions at which diamonds were first developed by the companies ASEA and General Electric in the early 1950s. Temperatures are in Kelvin--subtract 273 to transfer to degrees Celsius.
<< Back |
Next >>