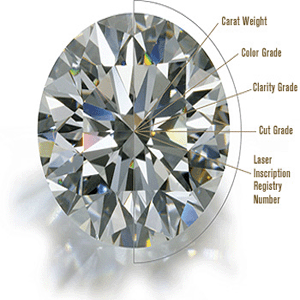
Home What is Diamond | Origins | History | Mining & Distribution | Diamond Jewels THE NATURE OF DIAMOND
Diamond, composed of carbon, is the hardest natural substance in the earth. Each carbon atom is enclosed by four neighboring carbon atoms in a tetrahedral coordination that is the result of a covalent bond and a face-centered arrangement in the cubic unit cell. Diamond is in the isometric crystal system, which is reflected in the usually found octahedral or cubic crystal form. The external crystal class is 4/mBar32/m, whereas the space group designation is F41dBar32/m. Twins are frequent on the {111} plane. It has perfect four directional cleavage, adamantine shine, and both a high refractive index, 2.42, and specific gravity, 3.52. Color is generally pale yellow to colorless, but can also be brown, blue, green, orange, red, and black. Diamond might be up to 3 billion years old, which is much older than their surface host rock (Harlow, 1998, p. 60). Diamond crystallization originates some 200 kilometers, or 320 miles, under the surface and the disaggregated crystals are merely transported to the surface via kimberlite and lamproite pipes (Harlow, 1998, p. 54). These igneous host rock formations are generally cylindrical in shape and act as a conduit from the Earth's mantle to the contintental crust. Diamonds are distinguished between various types, Ia, Ib, IIa, and IIb. Though this information is important to the diamond cutter, it is of no value to the student merely interested in diamond as a gemstone. Diamond's greater optical properties and hardness has earned this mineral the highest respect in both industry and jewelry. It has a long tradition of invincibility and therefore the Greek name, adamas. |